Views: 291
– Viruses that infect bacteria may one day replace antibiotics because they precisely attack only specific pathogens. Researchers at ETH Zurich are now showing that this is not always the case. This new finding is important because bacterial viruses can transfer antibiotic resistance genes.
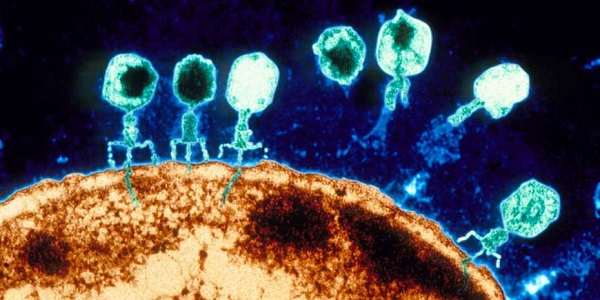
Courtesy ETH by Rahel Kunzler: Bacteriophages – phages for short – are viruses that only infect bacteria. To capture a bacterial host, they first bind to specific molecules on its cell surface. Then they inject their genetic material into the bacterial cell. To reprogram the bacteria’s cellular machinery to produce new viral particles, the phages also need to outsmart the target bacteria’s immune system.
Molecular entry points and the immune system differ from one bacterium to another, so it was commonly believed that most phages have a narrow host range – that is, they only infect a single bacterial species or even subspecies. This is also what led to the idea of using natural bacteria killers to treat infections – particularly when disease-causing bacteria have acquired resistance to antibiotics.
Now, however, a study led by Elena Gómez-Sanz, research associate in the group of Martin Loessner, professor of food microbiology at ETH Zurich, challenges the narrow-range phage host theory. Phages within the Staphylococcus group of bacteria usually infect several species simultaneously. The researchers recently published their results in the journal Nature Communicationscall_made.
“If we want to assess the role of phages as vectors of antibiotic resistance, we have to look at the whole picture – not just the situation in human medicine.” Elena Gómez-Sanz, Senior Scientist
Their findings could have direct consequences for phage therapy, which has not yet been approved for use in Switzerland but has long been in use in Eastern Europe. Phages not only kill bacteria, they can also transfer antibiotic resistance genes from one bacterium to another. Thus, their unexpectedly wide range of prey means that phages can spread their resistance genes much further into the environment than previously thought.
Many new phages isolated in wastewater
The mechanism by which phages can transfer antibiotic resistance between bacteria is already known. In short, when these viruses multiply in bacterial cells, they don’t just inject their own genetic material into new viral particles; in some cases, they smuggle genetic material from the infected bacteria – a resistance gene, for example – into the virus particles. If one of these virus particles infects a new bacterium, resistance can be transferred.
In staphylococci, this form of gene transfer has been particularly well studied. This group of bacteria, comprising more than 50 species, naturally colonizes not only humans but also livestock, and is found in natural water bodies. The best-known member of this group is Staphylococcus aureus – a bacterial species that naturally colonizes our nose and skin, but has recently become a dangerous multidrug-resistant pathogen.
Phages played a crucial role in the evolution of today’s most common and widespread multidrug-resistant pathogen, it is widely believed. Therefore, it is not surprising that more than 90% of the known phages in Staphylococcus originate from Staphylococcus aureus, usually isolated from clinical samples.
“However, if we want to assess the role of phages as vectors of antibiotic resistance, we have to look at the whole picture – not just the situation in human medicine,” says Gómez-Sanz.
Looking for the widest possible range of natural Staphylococcus phages, the ETH researchers visited wastewater treatment plants. After all, a great diversity of bacteria and their phages converge here – from the human microbiota, from livestock, from homes and industry. The researchers isolated a total of 94 phages from wastewater for their study.
Viruses encompass a giant network for gene transfer
The researchers performed laboratory experiments to identify the natural prey pattern of their isolated phages. To do this, they dropped the phages onto different potential host bacteria and studied their infection patterns. The 117 bacterial strains studied in total included representatives of 29 different species of Staphylococcus and bacteria from different habitats, with and without antibiotic resistance.
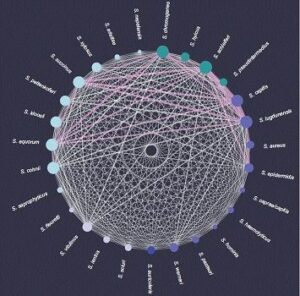
They found that a phage infects, on average, four different bacterial species. Or, from the bacteria’s point of view, “shared” phages can allow a staphylococcal species to exchange genetic material with, on average, more than 17 other species. “This huge network shows the tremendous impact that phages can have on bacterial communities,” says Gómez-Sanz.
The narrow host range hypothesis has likely survived for so long because there are almost no previous similar studies investigating phage infectivity in many different bacterial species, says the microbiologist. Previous work was often strictly limited to clinically relevant bacterial species such as S. aureus. However, the present study shows the urgent need to particularly investigate the prevalence of antibiotic resistance beyond the scope of individual biospheres.
“Human health is closely linked to the health of animals and the environment,” says Gómez-Sanz. The study highlights the importance of the modern “One Health Approach” in the use of antibiotics. New findings in the network of individual natural phages show, for example, that phages can transfer antibiotic resistance from bacteria in the animal microbiota directly to human pathogens. This underlines the importance of the modern “one health approach” to antibiotic use.
It is still unclear how often resistance genes spread
It is difficult to estimate from laboratory experiments carried out to date how often phages actually transfer antibiotic resistance genes in nature. However, the current study makes an assumption about which of the investigated phages are particularly potent vectors.
For 28 of the 94 phages, the ETH researchers investigated how often they can pick up a natural resistance gene while propagating the phages in a bacterium from the generated network. The frequency of absorption ranged from 1 in 100 particles to 1 in 10 million.
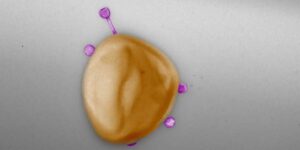
These huge differences are due to the different life cycles and enzymes that viruses use to package their genetic material. “Some are ‘more error prone’ than others, meaning they tend to include some bacterial genetic material in the package,” explains Gómez-Sanz. If, in addition, these types of phages have a wide host range, the risk of transfer is even greater.
As the authors emphasize, the study results are also significant with regard to fighting pathogenic bacteria in humans using phages. The discovery that phages can have a wide host range should be considered positive. This makes it easier to use them against countless different bacteria.
However, when using phages in medicine, care must be taken that they do not additionally act as vectors for antibiotic resistance genes. Therefore, it is important to ensure that the phages used in medicine have a propagation mechanism that works as perfectly as possible.
This work was supported by the national research program “Antimicrobial Resistance” NRP 72
Reference: Göller PC, Elsener T, Lorgé D, Radulovic N, Bernardi V, Naumann A, Amri N, Khatchatourova E, Coutinho F, Loessner MJ, Gómez-Sanz E: Multi-species host range of staphylococcal phages isolated from wastewater. Nature Communications 12: 6965, 2021. doi: 10.1038/s41467-021-27037-6
Related article: Bacteria-New antibiotic idea will prevent from sticking
Leave a Reply