Views: 57
– A vaccine candidate for COVID-19 has been identified by researchers from the Oxford Vaccine Group and Oxford’s Jenner Institute.
Courtesy Oxford University: Researchers at University of Oxford are working in an unprecedented vaccine development effort to prevent COVID-19 have started screening healthy volunteers (aged 18-55) today for their upcoming ChAdOx1 nCoV-19 vaccine trial in the Thames Valley Region. The vaccine based on an adenovirus vaccine vector and the SARS-CoV-2 spike protein is already in production but won’t be ready for some weeks still.
The team will enrol healthy volunteers aged between 18 – 55, who, if they pass screening, will be the first humans to test the new vaccine, called ChAdOx1 nCoV-19. The trial will provide valuable information on the safety aspects of the vaccine, as well as its ability to generate an immune response against the virus. Interested individuals can volunteer to participate on the COVID-19 vaccine website.
The trial, a collaboration between the University’s Jenner Institute and Oxford Vaccine Group clinical teams, will recruit up to 510 volunteers, who will receive either the ChAdOx1 nCoV-19 vaccine or a control injection for comparison. Whilst the team will start screening people now to see if they are eligible to take part in the study, participants will not receive the vaccine for some weeks. Detailed preclinical work is being done and the vaccine is being manufactured to clinical grade standard at the Clinical Biomanufacturing Facility at Oxford University. The trial has been approved by UK regulators and ethical reviewers. Researchers are working as quickly as possible to get the vaccine ready to be used in the trial, which includes further preclinical investigations and production of a larger number of doses of the vaccine.
Professor Adrian Hill, Director of the Jenner Institute at the University of Oxford, said, ‘The Oxford team had exceptional experience of a rapid vaccine response, such as to the Ebola outbreak in West Africa in 2014. This is an even greater challenge. Vaccines are being designed from scratch and progressed at an unprecedented rate. The upcoming trial will be critical for assessing the feasibility of vaccination against COVID-19 and could lead to early deployment.’
Professor Andrew Pollard, Chief Investigator on the study, said, ‘Starting the clinical trials is the first step in the efforts to find out whether the new vaccine being developed at Oxford University works and could safely play a central role in controlling the pandemic coronavirus that is sweeping the globe.’
Scientists around the world are working hard to develop a vaccine to prevent COVID-19, but there is a lot to be done. The Oxford team led by Professor Sarah Gilbert, Professor Andrew Pollard, Professor Teresa Lambe, Dr Sandy Douglas and Professor Adrian Hill started work designing a vaccine on Friday 10th January 2020.
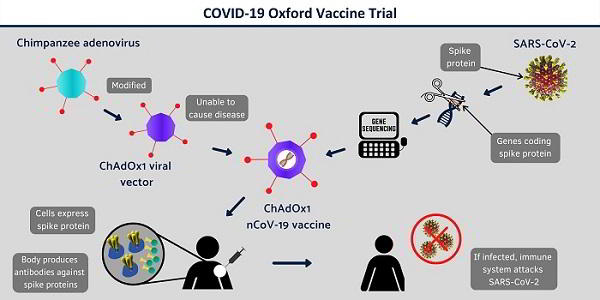
The vaccine is an adenovirus vaccine vector (ChAdOx1) and was developed at Oxford’s Jenner Institute. It was chosen as the most suitable vaccine technology for a SARS-CoV-2 (COVID-19) vaccine as it can generate a strong immune response from one dose and it is not a replicating virus, so it cannot cause an ongoing infection in the vaccinated individual. This also makes it safer to give to children, the elderly and anyone with a pre-existing condition such as diabetes. Adenoviral vectors are a very well-studied vaccine type, having been used safely in thousands of subjects, from 1 week to 90 years of age, in vaccines targeting over 10 different diseases.
Coronaviruses have club-shaped spikes on their outer coats. Immune responses from other coronavirus studies suggest that these are a good target for a vaccine. The Oxford vaccine contains the genetic sequence of this surface spike protein inside the ChAdOx1 construct. After vaccination, the surface spike protein of the coronavirus is produced, which primes the immune system to attack the coronavirus if it later infects the body. Professor Gilbert and team have previously developed a vaccine for another human coronavirus disease, which is Middle East Respiratory Syndrome (MERS), and this has shown promise in early clinical trials.
Professor Gilbert, lead researcher of the vaccine development programme, said, ‘Since the Ebola outbreak in West Africa in 2014, my research team has been working on new approaches to vaccine development to protect the population of the world against an outbreak of infectious disease or a pandemic. We are now working with a much larger team to bring these plans to fruition.’
At the same time as conducting the first clinical trial, production of the vaccine is being scaled up ready for larger trials, and potentially, future deployment. By starting vaccine manufacturing scale-up immediately, the team can ensure that enough vaccine doses are available as soon as possible – especially for NHS workers, the elderly, and those with underlying health conditions – if the trials prove that the vaccine is safe and effective.
Dr Sandy Douglas, who is leading on the vaccine manufacturing scale-up project, said, ‘The scale of this epidemic poses a huge challenge for vaccina manufacturing. We need to follow rigorous safety standards and that takes time. By starting work on large-scale manufacturing immediately, we hope to accelerate the availability of high quality, safe vaccina.’
Professor Teresa Lambe leading the early stages of our vaccine development said, ‘The commitment, compassion and helpfulness felt throughout the whole effort from everyone we have been working with has been amazing. We deeply appreciate the support of all our collaborators, funders and the teams around us in getting to this stage with the speed we have.’
Preclinical work on the ChAdOx1 nCoV-19 vaccine is being conducted in collaboration with our partners: Rocky Mountain Laboratories (NIH/NIAID); The ‘CSIROxbridge Consortium’; Public Health England; The Pirbright Institute; Professor Dr. Stephen Becker at the Institut für Virologie, Philipps‐Universität Marburg. Manufacturing is being conducted in collaboration with Prof Cath Green and the University of Oxford Clinical Biomanufacturing Facility, the Vaccine Manufacturing and Innovation Centre (Oxford), Advent Srl (Italy), Pall Biotech (Portsmouth), Cobra Biologics (Staffordshire), and Halix BV (Netherlands).
Participate in a Trial COVID-19 Vaccina Study
What is the purpose of this trial?
The purpose of this study is to test a new vaccine against COVID-19 in healthy volunteers.
A new virus causing respiratory disease emerged in Wuhan, China in December 2019 and has since rapidly spread to many other countries around the world, despite unprecedented containment efforts. The virus is part of the Coronavirus family which may cause respiratory infections ranging from the common cold to more severe diseases. This recently discovered coronavirus causes coronavirus disease COVID-19.
Common symptoms of COVID-19 include fever, tiredness, and dry cough. Whilst about 80% of infected people have only mild symptoms and will recover from the disease without needing special treatment, 1 in every 6 people who gets COVID-19 becomes seriously ill. Older people and those with underlying medical problems are more likely to develop serious illness. Thousands of deaths have been reported so far.
The WHO declared the COVID-19 epidemic a Public Health Emergency of International Concern on 30th January 2020. There are no currently licensed vaccinas or specific treatments for COVID-19. Vaccinas are the most cost effective way of controlling outbreaks and the international community have stepped-up their efforts towards developing one against COVID-19.
This study will enable us to assess if healthy people can be protected from COVID-19 with this new vaccina called ChAdOx1 nCoV-19. It will also give us valuable information on safety aspects of the vaccina and its ability to generate good immune responses against the virus. We will do this by randomly allocating participants to receive the vaccine or a placebo injection in addition to doing blood tests and collecting information about any symptoms that occur after vaccination.
Major information: Participate in a Trial
This study is a collaborative project which would not have been possible without the support of our partners. This work receives funding from the rapid research response funded by UK Research and Innovation (UKRI), and by the Department of Health and Social Care through the National Institute for Health Research (NIHR). The NIHR have also provided funding for the set-up of the project through the Oxford Biomedical Research Centre (BRC). The study has received funding from the Coalition for Epidemic Preparedness Innovations (CEPI) as part of an urgent call to expand the number of COVID-19 vaccine candidates in development. Funding has also been received from the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Science (CIFMS). The ‘CSIROxbridge Consortium’ (Principal Investigator Professor S. S. Vasan) is led by Australia’s science agency CSIRO for ‘High Containment Studies to Support Product Development’ for CEPI.
Source: University of Oxford
Related article: Development of mRNA-based coronavirus vaccine by CureVac