Views: 273
TU Berlin researchers are working on teeth developed from the body’s own material
Courtesy TU Berlin: Dr. Roland Lauster, professor of medical biotechnology at TU Berlin, explains what the project is about: “It’s true that there are isolated reports of people growing third teeth or even a third complete set of teeth, but why this should be possible for some people and not for others remains unknown.”
“Essentially science assumes that over the course of a lifetime the human jaw also possesses the information necessary for the growth of new teeth,” says Dr. Jennifer Rosowski, research assistant under Roland Lauster, whose doctoral thesis was on the topic of regrowing teeth. The question is what triggers this process.
Sharks do it, so do crocodiles and rodents and – theoretically – humans too. We are talking about regrowing teeth. Sharks don’t need to worry about losing teeth. If one falls out, another one will nearly always take its place. But what about people? If we lose our adult teeth, we need implants or dentures to be able to bite effectively.
Under natural conditions, hair, teeth, and even nails grow as a result of what is termed mesenchymal condensation. In the case of teeth, certain precursor cells cluster together in the jaw beneath the outer skin layer. These cells condense and form a kind of embryonic tooth germ. As a result of this condensation, the embryonic tooth germ begins to interact with surrounding cell layers in the jaw via specific messengers. “Within the tooth bud created by this process, a differentiation of various cell types occurs: the enamel organ, the dental papilla, and the dental lamina. These tissues continue to differentiate until a complete tooth is formed,” says Rosowski, describing the process of tooth development. The information determining which kind of tooth is required – incisor or molar – is provided by the surrounding jaw tissue.
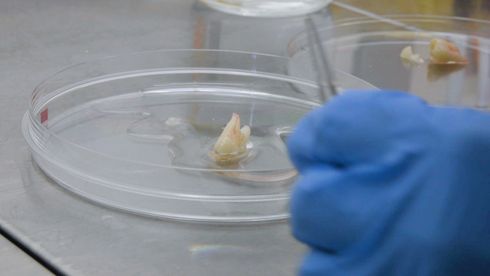
The approach adopted by researchers at TU Berlin for the natural growth of third teeth is as simple as it is ingenious: They remove dental pulp cells from the interior of an extracted tooth, which they then cultivate and de-differentiate in such a way as to produce an active embryonic tooth germ. If you were to implant this embryonic tooth germ into a patient, the idea goes, it would begin to communicate with the surrounding tissue, thus releasing the entire cascade of messengers and initiating the process of tooth development.
Competing research groups have already provided conceptual evidence in an animal model system and have demonstrated that an embryonic tooth implanted into the jaw actually develops into a complete tooth.

Roland Lauster’s team, however, sees a decisive competitive advantage in the method it has been pursuing: All other competing research groups use embryonic stem cells to produce embryonic tooth germs. “This makes a real application of the process impossible as the use of stem cells in most countries is ethically highly contentious and not permitted by law,” Jennifer Rosowski explains. “By contrast, we would only use cell material taken from the patient’s own teeth. This enables us to bypass all ethical and legal considerations, providing us with the decisive advantage that our procedures focus on an actual application of the body’s own tissue. Using the body’s own tissue means that no rejection reaction will occur.”
The department of oral surgery at Charité Universitätsmedizin Berlin provides the researchers with the teeth they require for their research in the form of removed wisdom teeth. The researchers have developed a special cultivation method to allow the adult cells in these wisdom teeth to de-differentiate back into a type of embryonic state and finally form an embryonic tooth germ. The dental pulp cells are isolated, cleansed, and then cultivated in microtiter plates whose upper surfaces have been coated with a hydrogel. The hydrogel prevents the cells becoming stuck to the walls of the titer plates. They float freely in the medium but are actually programmed to achieve a three-dimensional structure. This means that they are able to condense independently to form a type of cell ball, without any external pressure being applied. This process takes 24 hours and the resulting ball measures some 200 to 500 micrometers.
“We are the only group worldwide who have been able to demonstrate that this process of creating a ball through independent mesenchymal condensation triggers the expression of various genes, thus setting in motion the production of specific messengers. These messengers are required to interact with the surrounding jaw tissue,” Rosowski explains the process which has now been patented globally. To demonstrate this “inductance”, the researchers co-cultivated the embryonic tooth germs together with cells from gums. During embryonic tooth development, these two types of cells interact, initiating the further development of the tooth. And it is this interaction which the TU researchers have succeeded in demonstrating.
Now that all the in-vitro tests have been successfully completed, the embryonic tooth germs are ready for the first pre-clinical tests.
For further details, please contact:
Prof. Dr. Roland Lauster TU Berlin
Department of Medical Biotechnology
Leave a Reply