Views: 47
Personalized heart computer models pave the way for personalized therapies – the risk of cardiac arrhythmias, such as atrial flutter, can now be assessed individually.
Article Courtesy by KIT: Digital human organ simulations make it possible to explore disease development and personalized therapies for patients. At the Karlsruhe Institute of Technology (KIT), researchers are developing realistic computer models of the heart at various levels: from the ion channel via cells and tissues to the entire organ. They simulate basic physiological and pathological processes, but also develop custom models to individually assess the risk of cardiac arrhythmias such as atrial flutter and the effects of therapies, as reported in a journal.
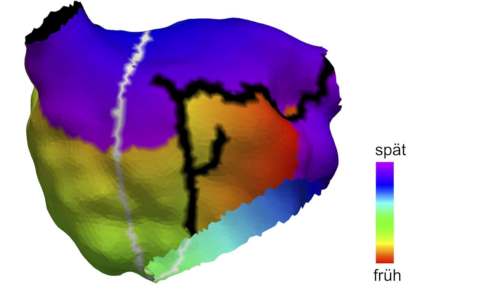
How high the risk of a patient developing atypical atrial flutter has not been reliably assessed. Researchers at the Karlsruhe Institute of Technology, Medical Clinic IV, Karlsruhe Municipal Clinic and the Faculty of Medicine of the University of Freiburg and the Freiburg University Cardiac Center – Bad Krozingen have now developed a method for assessing the risk of atrial flutter individually: Scientists at The Frontiers of Physiology report that custom computer models allow us to identify all the ways in which atypical and circulating electrical impulses can occur. “Our models include anatomical, electrophysiological and pharmacological criteria,” explains Dr. Axel Loewe, Head of the Heart Modeling working group at the KIT Institute of Biomedical Engineering. The effect of therapies such as catheter ablation or medication can therefore be assessed individually in advance.
The work demonstrates the advantages of mathematically simulated organs for medicine: “Computer models provide a perfectly controllable experiment environment,” Loewe explains. “In this way, individual changes can be simulated and their consequences calculated for the entire system.” The models complement classical methods, such as experiments with cells and animals, and enable new therapies to be tested without risk to humans.
Already in his dissertation, Loewe simulated the causes of atrial fibrillation with the computer. Heart Modeling’s KIT working group, led by Loewe, develops realistic models of the heart at all levels, from the ion channel through cells and tissues to the entire organ. In this way, they can simulate how an electrical arousal develops, spreads through the atria and throughout the heart, and – in the case of a healthy beating heart – goes out or – in the case of certain cardiac arrhythmias – sustains itself permanently.
In addition to simulating such basic physiological and pathological processes, the group also works with custom models to individually determine disease risk and the effect of treatments. To capture personal anatomy, such as the size and shape of a patient’s atria, researchers use imaging techniques such as magnetic resonance imaging. By integrating the electrical activity of the heart’s electrocardiogram (ECG), the group works closely with the KIT Institute of Biomedical Engineering, led by Professor Olaf Dössel. The work that moves between engineering, computer science, science and medicine,
An atrial flutter is a cardiac arrhythmia in which unusually rapid electrical arousal patterns cause the atria to contract rapidly. Unlike most common atrial fibrillation, electrical excitation is coordinated in the atrial flutter. But, like atrial fibrillation, atrial vibration leads to palpitations, breathlessness, and weakness; The risk of stroke is also increased. A typical treatment of fibrillation is ablation, which is catheter-assisted sclerotherapy of morbid electrical sources of excitement in myocardial tissue. Frequently, however, patients develop after treatment the so-called atypical atrial flutter, in which circular excitation may occur in the left atrium and the right atrium.
Original Publication (Open Access):
Axel Loewe, Emanuel Poremba, Tobias Oesterlein, Armin Luik, Claus Schmitt, Gunnar Seemann and Olaf Dössel: Patient Specific Identification of Atrial Flutter Vulnerability – A Computational Approach to Reveal Latent Reentry Paths.
Frontiers in Physiology, 2019. DOI: 10.3389 / fphys.2018.01910 https://www.frontiersin.org/articles/10.3389/fphys.2018.01910/full
Caption picture above: Anatomical model of the left atrium of a 70-year-old female patient. Black: Existing ablation scars from previous treatment. Gray: Path identified by the algorithm along which atrial vibration may arise. Color code: clinically measured activation time of the atrial flutter (Figure: Axel Loewe, KIT)
Related article: Automating artificial intelligence for decision-making